Aspartic Acid at Ph 3
Aspartic acid is one of the α-amino acids that are produced when Protein is being hydrolysed. It is a β-amino acid with the side chain carboxyl moved to the backbone.
A Which structure shows the predominant form of aspartic acid at pH 3.

. The energetics of a salt bridge formed between the side chains of aspartic acid 70 Asp70 and histidine 31 His31 of T4 lysozyme have been examined by nuclear magnetic resonance techniques. You have to know what kind of group is associated with each. 2015 a I b II c III d IV e none of these choice.
63 x 10 Submit. The novelty of the prepared hydrogels is based on the incorporation of pH-sensitive monomer acrylic acid with polymer aspartic acid in the presence of ethylene glycol dimethacrylate. The aspartic acid formula looks like this- C 4 H 7 NO 4.
How do you calculate an isoelectric point with 3 pKa. Degradative pathway contributing to the chemical instability of peptides and proteins at neutral to slightly alkaline pH. Isoaspartic acid isoaspartate isoaspartyl β-aspartate is an aspartic acid residue isomeric to the typical α peptide linkage.
At a pH below its pKaside chain the sulfhydryl. A 60 b 69 c 53 d 30 e none of these choices. Monoisotopic mass 133037506 Da.
Aspartic acid is an example of alpha amino acid dicarboxylic amino acid with acidic character. For aspartic acid at pH 7 what is the ratio of the concentration of the neutral amino acid species to the negatively charged species zwitterion backbone and deprotonated side chain. A H H-N CH-C -0 H CH2 -o b H H Н CH C OH H CH ОН.
Aspartic acid has an average occurrence of about 5 in all proteins so Asp-N is widely used in peptide mapping and proteomic analysis. Aspartic Acid is a one of the non-essential amino acids that forms the basic building block of a very important biomolecule ProteinAspartic Acid like all other amino acids contains amino -NH 2 group as well as carboxyl -COOH groups in its structure. Just knowing the pKa values isnt enough.
An aspartic acid structure is no exception. Average mass 133103 Da. When the pH is sufficiently low the acid groups of both the native and unfolded states are fully protonated and the apparent unfolding equilibrium.
Encoded by the codons GAU and GAC is an ɑ-amino acid that is used in the biosynthesis of proteins. Due to the pH-sensitive nature the release of acetaminophen was prolonged for an extended period of time by the developed hydrogels. COO- form under biological conditions and a side chain CH 2 COOH classifying it as a chargedat physiological pH aliphatic amino acid.
All pKa values can be found in Table OA. Draw the form of the substance with all the protonatable groups protonated. Under physiological condition pH 74 the amino group NH 2 group is protonated and becomes NH 3 but carboxylic acid group COOH group is deprotonated and present as COO.
ABSTRACT The racemization half-lives ie the time required to reach a DL 033 at pH 68 for aspartic acid and phenylalanine in the sweetener aspartame L-aspartyl. Aspartic acid abbreviated as Asp or D. Which of the structures in Figure 1 shows the prevalent form of aspartic acid at pH 3.
- 1 of 1 defined stereocentres. We offer a huge of aspartic acid at ph 3 news and articles here. Given pKa values of 21 98 and 39 side chain what is the pI of aspartic acid.
Carboxylic acids would be COOH. Which structure shows the predominant form of aspartic acid at pH 3. The ionic form of this amino acid is known as aspartate.
Chemistry questions and answers. It contains one α-amino group which is in NH 3 forms and the carboxylic group that is in deprotonated COO-. It is semi-essential in humans meaning the body can.
Aspartic acid is a non-essential amino acid as. Such a change is caused by a chemical reaction in which the nitrogen atom on the N1 following peptide bond in black at top right of Figure 1 nucleophilically attacks the γ-carbon of the. 13 x 10-6 C.

Molecular Structure Of Aspartic Acid Glycine And Lysine With Pk A Download Scientific Diagram

Scheme 1 Structural Formula Aspartic Acid Asp In Its Zwitter Ionic Download Scientific Diagram
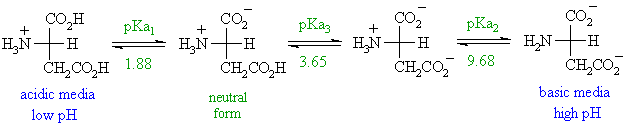
No comments for "Aspartic Acid at Ph 3"
Post a Comment